Supercritical fluid extraction (SFE) using CO2 as the sole solvent is one of the most widely used green extraction technologies. This method is especially effective for extracting non-polar, low molecular weight, lipophilic compounds that are either highly soluble or miscible with supercritical CO2 (as defined by solubility rules ② and ③).
Compounds that fall under categories ⑤ and ⑥ are difficult to extract and typically remain in the residue. Research shows that pure supercritical CO2 often extracts volatile oils, fats, alcohols, ethers, esters, and resins. Because the process occurs near room temperature, it avoids degradation of thermally sensitive components and eliminates solvent residue—making it ideal for preserving the natural quality of the extract.
Raw Material Preparation and Process Optimization
To achieve optimal extraction results, the following factors must be considered:
- Raw Material Pre-treatment: Grinding, slicing, particle size, and moisture content
- Extraction Parameters: Pressure, temperature, duration, solvent-to-material flow ratio, CO2 flow rate
- Separation Parameters: Separator pressure and temperature, phase separation efficiency, solvent recovery, and recycling
The cycle duration of supercritical fluid circulation depends on solute extraction capacity and separation factors. Laboratory studies typically use methods like response surface methodology (RSM), single-factor analysis, or least squares regression to optimize for yield and selectivity.
Case Study: Clove Oil Extraction

Researchers compared several methods for extracting clove oil, revealing the advantages of supercritical CO2:
| Extraction Method | Yield (%) | Eugenol (%) | Acetyl Eugenol (%) | Time (h) | Color & Texture | Solvent Used | Aroma |
|---|---|---|---|---|---|---|---|
| Supercritical CO2 | 19.6 | 58.8 | 19.6 | 2 | Pale yellow oil | No | Strong |
| Steam Distillation | 10.1 | 61.2 | 10.2 | 8–10 | Pale yellow oil | Yes | Strong |
| Water Distillation | 11.5 | 50.3 | 3.2 | 4–6 | Dark brown oil | Yes | Strong |
| Soxhlet Extraction | 41.8 | 30.8 | 9.3 | 6 | Brown extract | Yes | Weak |
SFE offered the highest quality with shorter extraction time and no solvent residues.
Multi-Stage Extraction and Separation
Multi-stage operations are often used to improve selectivity:
- Multi-Stage Extraction: Pressure is increased step-by-step to extract components in order of polarity, boiling point, and molecular weight
- Multi-Stage Separation: Different separators operate at various pressures and temperatures to selectively precipitate compounds
Example:
Stage 1 (30 MPa, 60°C): Extract contains volatiles, esters, free fatty acids, fats, waxes, resins, and pigments
Stage 2 (10 MPa): Waxes, pigments, and resins precipitate in the first separator
Stage 3 (6 MPa): Esters, fatty acids, and fats dominate the second separator
Stage 4 (<6 MPa): Volatile aromatic oils are recovered in the third separator
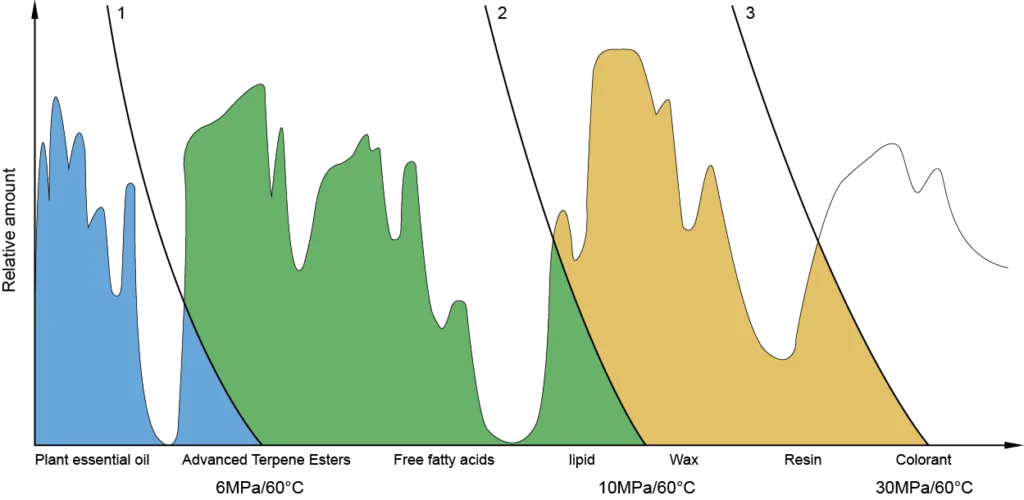
These fractions can be blended to meet specific product standards. For example, in sage oil extraction, a two-stage system was used to separate waxes in separator II (8.5 MPa) and capture volatile oil in separator I.
Solubility Rules in Supercritical CO2
Understanding solubility behavior is critical for effective extraction:
- ①Solubility changes continuously between subcritical and supercritical states
- ②CO2 has a strong homogenizing power and can form homogeneous phases with over 140 non-polar or weakly polar compounds
- ③Solubility decreases as carbon chain length and molecular weight increase
- ④Strongly polar groups (e.g., –OH, –COOH) significantly reduce solubility
- ⑤Inorganic salts, sugars, amino acids, starches, and proteins are almost insoluble in CO2
- ⑥Compounds with a molecular weight above 500 Da are nearly insoluble
These rules help determine if co-solvents are needed and guide solvent selection.
FAQ
Q1: What compounds can be extracted with pure CO2?
A1: Lipids, essential oils, esters, alcohols, and other low-polarity, small molecules.
Q2: Why is supercritical CO2 extraction considered eco-friendly?
A2: It avoids toxic solvents, operates at mild temperatures, and leaves no harmful residues.
Q3: Can CO2 extract polar substances?
A3: Not effectively; co-solvents are usually needed for polar compounds.
Q4: What are the typical conditions for CO2 extraction?
A4: Generally 35–60°C temperature and 20–30 MPa pressure, depending on the material.
Q5: How does multi-stage separation improve product quality?
A5: It allows selective removal of impurities like waxes and pigments, producing purer fractions.
Trusted by Global Clients
Our CO2 extraction systems have been sold to over 30 countries, including the USA, Germany, Thailand, and Australia. From start-up brands to well-known herbal extract manufacturers, our customers trust us for:
- Reliable equipment performance
- Excellent technical support
- One-stop turnkey solutions
- Remote installation guidance and training
Supercritical CO2 Extraction Market Report
Contact Us
Looking for the right supercritical CO2 extraction equipment for your business? Contact us today for custom quotes, technical consultation, and free extraction process evaluation.
Email: [email protected]
WhatsApp/Phone: +86-134-8515-5519
You can view other models of supercritical CO2 extraction equipment [View More]