Mint (Mentha haplocalyx Briq.), a Lamiaceae herb, is widely used in traditional medicine for relieving wind-heat, clearing the head, and supporting the liver. Its main components include volatile oils, flavonoids, phenolic acids, and anthraquinones. Modern research highlights flavonoids like hesperidin, luteolin glycoside, and diosmin for their antioxidant, anti-inflammatory, and antiviral properties.
Traditional extraction methods, such as those using organic solvents and ultrasound, are less efficient and less environmentally friendly. Supercritical CO2 extraction offers a clean and efficient solution, ideal for thermolabile compounds like flavonoids. This study focuses on optimizing the supercritical CO2 method to improve yield and purity for industrial use.

1. Materials and Methods
1.1 Instruments and Equipment
- 1L SC-CO2 extraction system
- UV–Vis spectrophotometer
- Rotary evaporator
- Precision balance
- Ultrasonic cleaner

Learn about other best-selling models of supercritical CO2 extraction equipment [Read More]
1.2 Materials and Reagents
- Dried, ground mint
- CO2 gas (≥99.5%)
- Ethanol (various concentrations)
- Rutin (for standard curve)
- Petroleum ether (30–60 °C), aluminum nitrate, sodium nitrite, sodium hydroxide
- Ultrapure water
(All reagents were of analytical grade.)
2. Experimental Procedure
2.1 Extraction of Total Flavonoids
100 g of mint powder was loaded into the vessel. CO2 and ethanol entered under supercritical conditions (>31.1 °C, >7.38 MPa). The extract was concentrated, defatted with petroleum ether, and redissolved in 70% ethanol.
2.2 Rutin Standard Curve
0.0121 g of rutin was dissolved in 70% ethanol (0.2 mg/mL). Reacted with sodium nitrite, aluminum nitrate, and sodium hydroxide, then diluted. Absorbance at 510 nm gave:
A = 14.859C – 0.056 (R² = 0.9993)
2.3 Yield Calculation
Flavonoid content in extracts was measured using the same method.
Yield (%) = (C × V × dilution factor) / (m × 10⁻³) × 100%
Where:
•C = concentration (mg/mL)
•V = volume (mL)
•m = sample weight (g)
3. Results and Discussion
3.1 Effect of Co-solvent Type
Flavonoids are poorly soluble in pure CO2. Ethanol (50%–90%) was tested. 70% ethanol gave the highest yield (3.62%), showing it best matched flavonoid polarity.
3.2 Single-Factor Analysis
- Ethanol Dosage: Best yield at 6 mL/g. Higher amounts gave no clear advantage. (See Figure 1 below)
- Pressure: 25 MPa optimized yield, safety, and cost. (See Figure 2 below)
- Temperature: 55 °C gave the highest yield. Higher temperatures caused degradation. (See Figure 3 below)
- Time: 90 minutes was optimal. Longer times didn’t improve results. (See Figure 4 below)
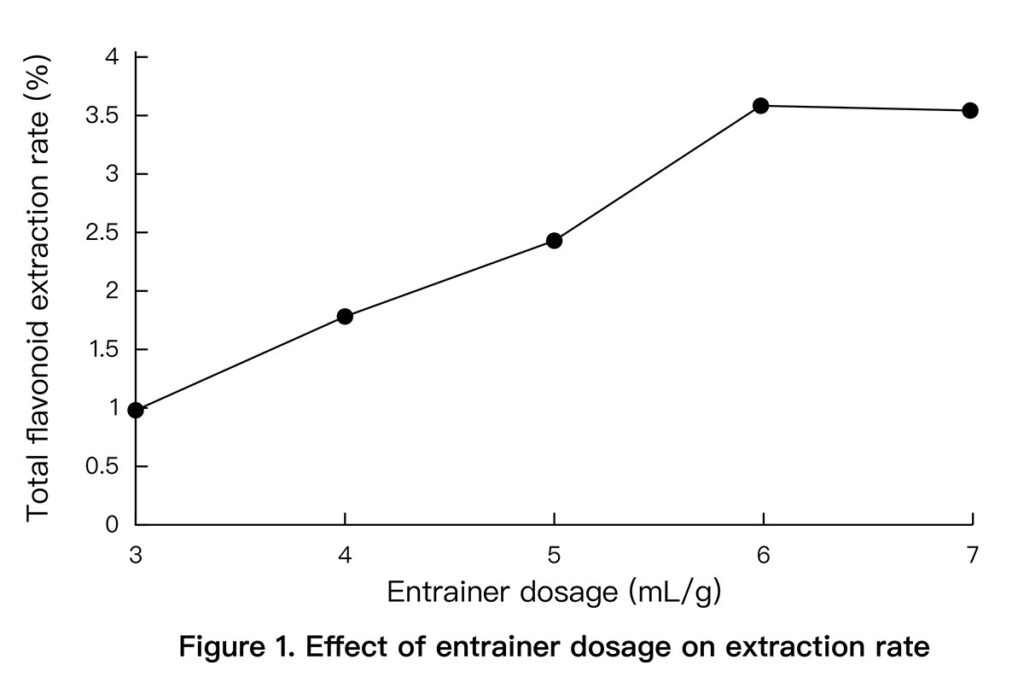
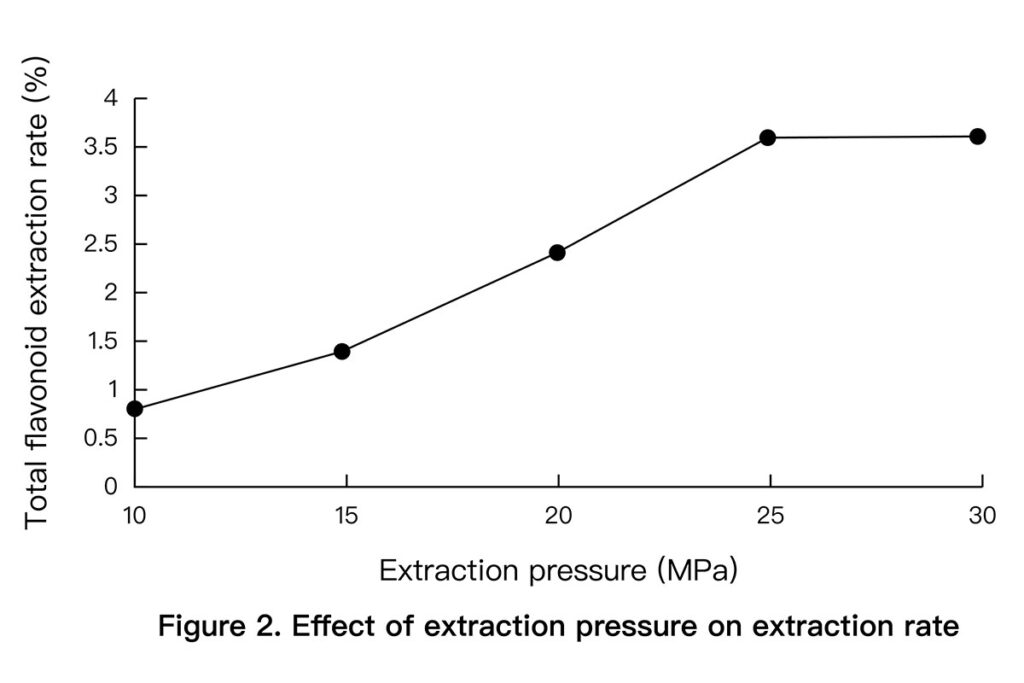
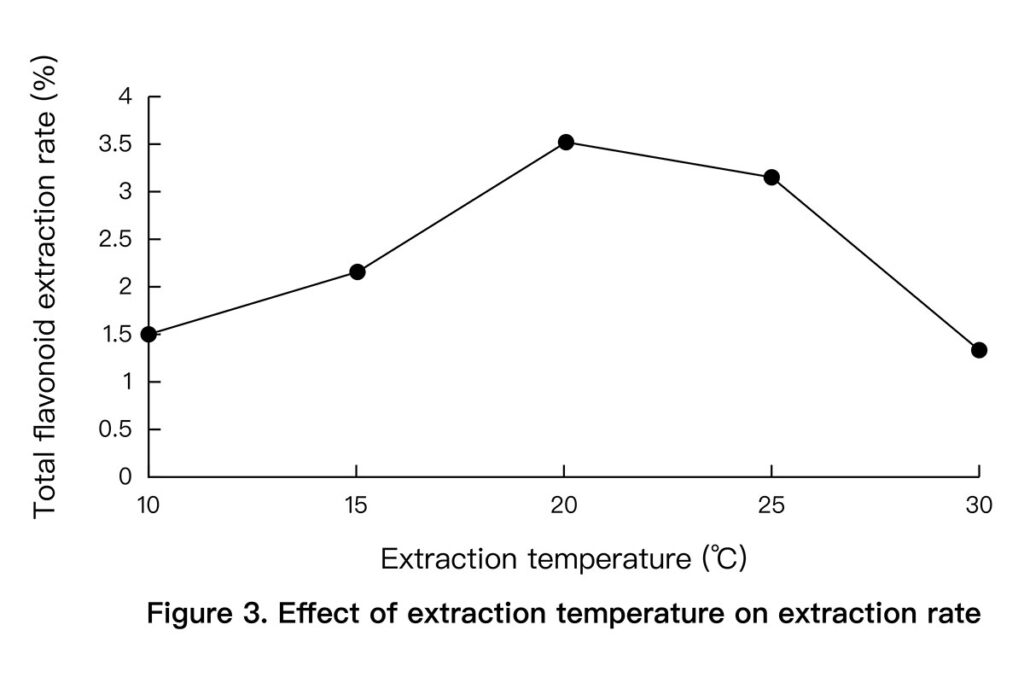
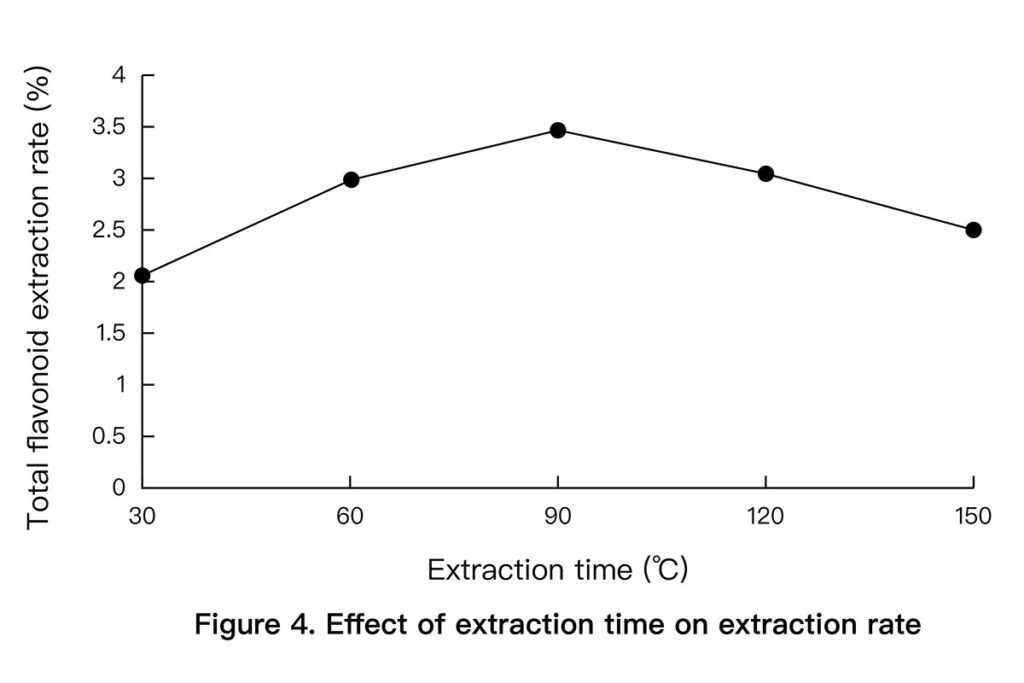
3.3 Orthogonal Optimization
An L9 (3⁴) orthogonal design tested interaction effects. Factor importance:
Ethanol dosage > Pressure > Time > Temperature
Best condition (A3B3C2D2) gave 3.58% average yield. Results were consistent across trials.
4. Conclusion
Mint is rich in flavonoids with proven health benefits. Supercritical CO2 extraction with ethanol enhances efficiency, selectivity, and product purity. Controlling key variables—especially ethanol concentration, pressure, and temperature—is essential.
Final Optimized Parameters:
- Ethanol concentration: 70% at 6 mL/g
- Pressure: 25 MPa
- Temperature: 55 °C
- Time: 90 minutes
This method is efficient, reproducible, and scalable for the pharmaceutical, nutraceutical, and food industries.
Frequently Asked Questions (FAQ)
Q1: Why use supercritical CO2 extraction for mint flavonoids?
It’s clean, solvent-free, and ideal for sensitive compounds, offering high purity and safety.
Q2: Why add ethanol?
Ethanol improves CO2 polarity, enhancing the extraction of polar compounds like flavonoids.
Q3: Is this method suitable for industry?
Yes. The optimized process is consistent and scalable for commercial production.
Q4: How does pressure affect extraction?
Higher pressure improves CO2 density and solubility, but going too high adds cost without much gain.
Q5: Does a higher temperature help?
Only up to a point. High heat boosts solubility but may damage sensitive flavonoids.
Trusted by Global Clients
Our CO2 extraction systems have been sold to over 30 countries, including the USA, Germany, Thailand, and Australia. From start-up brands to well-known herbal extract manufacturers, our customers trust us for:
- Reliable equipment performance
- Excellent technical support
- One-stop turnkey solutions
- Remote installation guidance and training
Supercritical CO2 Extraction Market Report
Contact Us
Looking for the right supercritical CO2 extraction equipment for your business? Contact us today for custom quotes, technical consultation, and free extraction process evaluation.
Email: [email protected]
WhatsApp/Phone: +86-134-8515-5519