Discover how solubility in supercritical CO2 impacts extraction efficiency. Learn about influencing factors, measurement methods, prediction models, and industrial applications in this in-depth guide.
Introduction to Solubility in Supercritical CO2
Understanding the solubility in supercritical CO2 is essential for optimizing extraction efficiency and selectivity in natural product processing and green chemistry. The solubility of solutes in SC-CO2 depends on several key factors such as pressure, temperature, molecular polarity, and the use of co-solvents. This article explores how each of these parameters influences SC-CO2 solubility and how predictive models like the Chrastil equation can guide process design in supercritical CO2 extraction systems.
What Is Solubility in Supercritical CO2?
Solubility in supercritical CO2 refers to the amount of a substance (solute) that can be dissolved in CO2 when it is in its supercritical state, above its critical temperature (31.1 °C) and critical pressure (7.38 MPa). This property plays a vital role in supercritical CO2 extraction because it determines how efficiently and selectively target compounds can be extracted from raw materials.
Link: Solubility in supercritical carbon dioxide
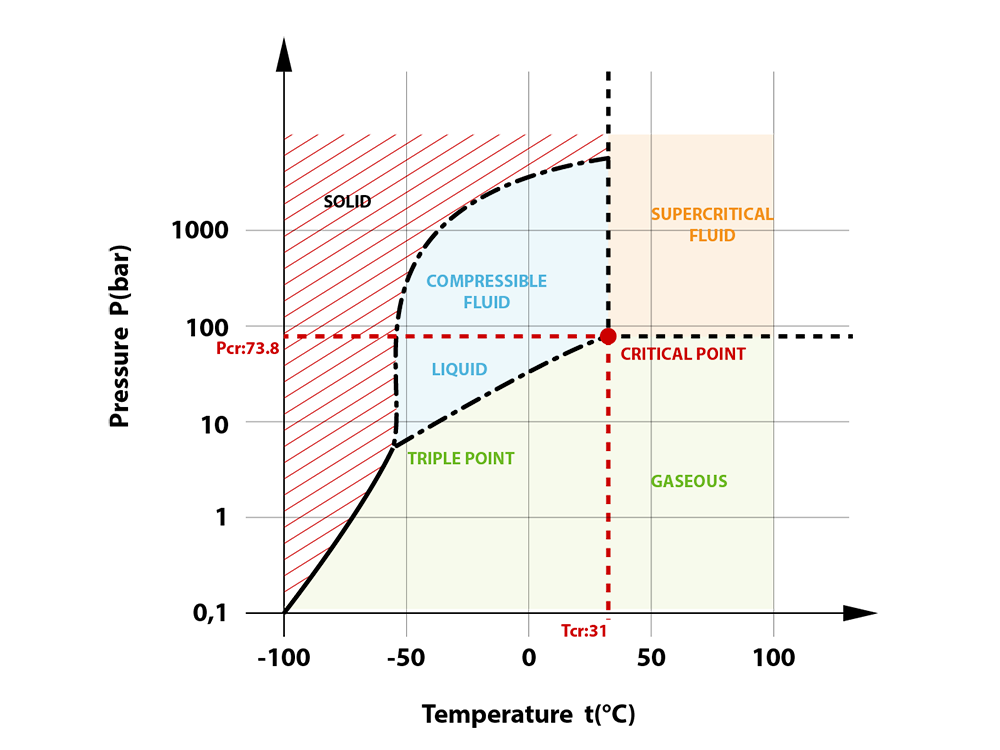
Key Factors Affecting Solubility
- Pressure Effects on Solubility in Supercritical CO2. One of the most influential parameters is pressure. As pressure increases, the density of supercritical CO2 also increases, which enhances its solvating power. Generally, higher pressures result in higher SC-CO2 solubility, but excessive pressure may reduce energy efficiency and increase equipment costs.
- Temperature Considerations Temperature affects both the vapor pressure of the solute and the density of CO2. Higher temperatures may increase solute volatility but decrease CO2 density. The net effect on solubility depends on the compound and typically exhibits a non-linear trend known as the “crossover effect.”
- Solute Polarity and Molecular Structure Non-polar and low-polarity compounds, such as lipids, terpenes, and waxes, usually exhibit better solubility in SC-CO2. In contrast, polar compounds like alkaloids or polyphenols may have limited solubility unless co-solvents are used.
- Role of Co-Solvent in Supercritical Fluids The addition of a polar co-solvent in supercritical fluids, such as ethanol, significantly enhances the solubility of polar solutes. This technique is common in botanical extractions and pharmaceutical applications to broaden the solute range and improve yield.

Extended Analysis: Solute Polarity as a Determining Factor
Why is polarity important?
Supercritical CO2 (SC-CO2) is a non-polar or weakly polar solvent, meaning it preferentially dissolves substances with similar polarity. This follows the classical chemical rule:
“Like dissolves like.”
Non-polar solvents tend to dissolve non-polar or weakly polar solutes more effectively, while strongly polar compounds are typically insoluble or poorly soluble in SC-CO2.
Understanding the polarity of the target solute is, therefore, a primary consideration in determining whether SC-CO2 is a suitable extraction solvent.
How to assess solute polarity?
Polarity can be assessed through several parameters:
| Evaluation Method | Description | Example |
|---|---|---|
| Molecular structure | Presence of polar groups (–OH, –COOH, –NH₂) indicates high polarity | Caffeine (weakly polar) vs Glucose (polar) |
| LogP value (partition coefficient) | LogP > 1 suggests low polarity, better CO2 solubility | Vitamin E: LogP ≈ 9.5 (highly soluble) |
| Water solubility | Strong water solubility indicates high polarity | Essential oils are non-polar and hydrophobic |
| Literature precedent | Reference to SC-CO2 extraction studies can confirm compatibility | Squalene, capsaicin, essential oils |
Case Analysis: Examples of Polarity vs CO2 Solubility
| Compound | Polarity | SC-CO2 Suitability | Reason |
|---|---|---|---|
| Rose essential oil (terpenes) | Non-polar | Excellent | Dissolves easily in SC-CO2 |
| Caffeine | Weakly polar | Conditional | Soluble under higher pressure or with modifiers |
| Polyphenols | Moderate–high | Limited | Requires co-solvent like ethanol |
| Glucose | Strongly polar | Not suitable | Insoluble in SC-CO2 |
| Vitamin E | Non-polar | Ideal | High solubility confirmed in many studies |
Use of Co-solvents for Moderately Polar Solutes
When the solute is moderately polar, a co-solvent (modifier) like ethanol, methanol, or acetone can be added to enhance the polarity of SC-CO2, improving solubility.
| Scenario | Co-solvent Recommendation |
|---|---|
| Extracting essential oils/fats | Not needed |
| Extracting flavonoids/polyphenols | Use 5–15% ethanol |
| Extracting sugars/proteins | Ineffective |
Summary: Polarity and Solubility Matching Table
| Polarity Type | Example Compounds | SC-CO₂ Suitability |
|---|---|---|
| Non-polar | Sugars, amino acids, and proteins | Highly suitable |
| Weakly polar | Caffeine, capsaicin, coumarin | Conditional |
| Moderate | Flavonoids, some polyphenols | Co-solvent needed |
| Strongly polar | Sugars, amino acids, proteins | Not recommended |
Methods to Measure and Predict Solubility
Experimental Techniques
Several methods are used to measure solubility in supercritical CO2, including:
- Static equilibrium method: Measures solubility after reaching a stable CO2–solute equilibrium.
- Dynamic flow method: CO2 is passed through the solute and concentrations are measured at the outlet.
- Analytical methods: GC, HPLC, or MS are used to quantify solute content in CO2.
Chrastil Model: Predicting Solubility in SC-CO2
The Chrastil model is a widely used empirical equation that predicts solubility based on temperature and CO2 density:
ln(S) = k * ln(ρ) + a / T + b
Where:
- S = solubility,
- ρ = CO2 density,
- T = temperature (K),
- k, a, b = model constants for a given solute.
This model helps estimate how changes in process parameters affect solubility and extraction efficiency.
Industrial Applications of SC-CO2 Solubility
Understanding SC-CO2 solubility is crucial in designing scalable and efficient extraction systems:
- Process Optimization: Solubility data helps define ideal pressure–temperature settings for target compounds.
- Selective Extraction: Use solubility differences to isolate specific fractions (e.g., essential oils vs waxes).
- Cost Reduction: Avoid over-pressurization and unnecessary energy consumption by using solubility-informed design.
- Wider Applications: Improves extraction in industries such as food, cosmetics, nutraceuticals, and traditional medicine.
Conclusion
Solubility in supercritical CO2 is a fundamental property that determines the success and efficiency of SC-CO₂ extraction processes. By understanding how pressure, temperature, solute structure, and co-solvents affect solubility—and by applying predictive models like the Chrastil equation—engineers and researchers can design greener, more effective extraction systems for a wide range of natural products.
FAQ Section (Frequently Asked Questions)
❓ What affects solubility in supercritical CO₂ the most?
Answer: The most influential factors are pressure, temperature, solute polarity, and the use of co-solvents. Among these, pressure plays a dominant role as it directly affects the density—and thus solvating power—of CO₂.
❓ How can I increase the solubility of polar compounds in SC-CO₂?
Answer: By adding a co-solvent, such as ethanol or methanol. These polar modifiers enhance the solvency of supercritical CO₂ and allow extraction of polar bioactives, such as flavonoids or alkaloids.
❓ Is the Chrastil model accurate for all solutes?
Answer: The Chrastil model provides good approximations for many solutes, especially non-polar compounds, but may not be highly accurate for strongly polar or thermolabile compounds. For complex systems, other models like the Mendez-Santiago-Teja-Teja or PC-SAFT may be more suitable.
❓ Why is understanding solubility important in supercritical CO2 extraction?
Answer: Because solubility directly impacts extraction yield, selectivity, process time, and cost efficiency. Optimizing for solubility helps avoid under- or over-processing, ensuring cleaner and more consistent extracts.
Trusted by Global Clients
Our CO2 extraction systems have been sold to over 30 countries, including the USA, Germany, Thailand, and Australia. From start-up brands to well-known herbal extract manufacturers, our customers trust us for:
- Reliable equipment performance
- Excellent technical support
- One-stop turnkey solutions
- Remote installation guidance and training
Supercritical CO2 Extraction Market Report
Contact Us
Looking for the right supercritical CO2 extraction equipment for your business? Contact us today for custom quotes, technical consultation, and free extraction process evaluation.
Email: [email protected]
WhatsApp/Phone: +86-134-8515-5519
You can view other models of supercritical CO2 extraction equipment [View More]