Entrainer (co-solvent) Introduction
Supercritical CO2 extraction is a widely used green technology for extracting essential oils, lipophilic compounds, and active ingredients from natural products. However, due to its non-polar nature, pure CO2 is limited to efficiently extracting low-polarity, low-molecular-weight substances. This limits its selectivity and effectiveness, especially for polar compounds.
To overcome this challenge, researchers and industry professionals often incorporate entrainers (also called co-solvents or modifiers) into the supercritical CO2 (SC-CO2) system. These additives significantly improve solubility and enhance extraction selectivity, expanding the range of extractable compounds and enabling more precise targeting of active ingredients.
Further reading: How can I increase the solubility of polar compounds in SC-CO₂?
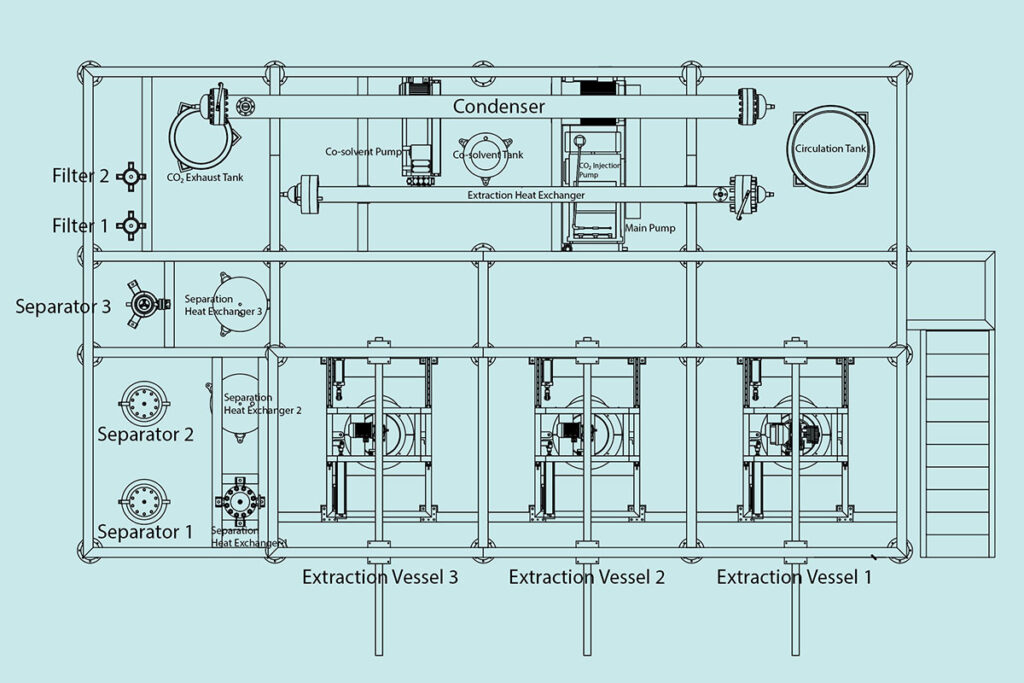
What Is an Entrainer in Supercritical CO₂ Extraction?
An entrainer is a small amount of polar or non-polar solvent introduced into the SC-CO₂ system to boost its ability to dissolve solutes. The entrainer interacts with the solute through mechanisms such as hydrogen bonding, van der Waals forces, and Lewis acid-base interactions, making it especially effective for polar or thermally sensitive molecules.
There are two types of entrainer systems:
- Miscible supercritical solvents, such as C₃–C₅ alkanes (e.g., propane, pentane)
- Subcritical organic solvents, such as ethanol or acetone, are added to supercritical CO₂ to form a single-phase or dual-phase mixed fluid
Key Benefits of Using Entrainers
- Increased Solubility
- Entrainers significantly raise the solubility of target compounds in SC-CO₂, especially those with polar functional groups.
- Improved Selectivity
- Target solutes can be selectively extracted due to specific molecular interactions, allowing for purification and fractionation.
- Reduced Operating Pressure
- With entrainers, effective extraction can occur at lower CO₂ pressures, reducing equipment stress and energy costs.
- Expanded Applicability
- Enables the extraction of bioactive molecules such as polyphenols, flavonoids, and alkaloids that are otherwise poorly soluble in pure CO₂.
Entrainer Selection and Properties
Entrainer choice is critical and should consider:
- Polarity matching with the target solute
- Low toxicity and easy removal from the final product
- Regulatory compliance for food, cosmetic, or pharmaceutical use
Below are common entrainers and their physical properties:
| Solvent | Formula | Dipole Moment (D) | Solubility Parameter (δ, J/m³)¹⁄² |
|---|---|---|---|
| Methanol | CH₃OH | 1.70 | 40.5 |
| Ethanol | C₂H₅OH | 1.69 | 37.0 |
| Acetone | C₃H₆O | 2.91 | 28.0 |
| Benzene | C₆H₆ | 0.0 | 20.7 |
| Cyclohexane | C₆H₁₂ | 0.0 | 16.8 |
Ethanol is often the most preferred entrainer for natural product extraction due to its GRAS status, low toxicity, and polarity compatibility.
Static vs. Dynamic Entrainer Addition Methods
- Static method: Entrainer is pre-mixed with raw materials before CO₂ introduction; suitable when solute-matrix interaction is strong.
- Dynamic method: Entrainer is continuously added via high-pressure pumps; preferred for higher solubility control and yield.
Case Study: Enhancing the Extraction of Salvia miltiorrhiza
Salvia miltiorrhiza, also known as Danshen, is a traditional Chinese medicinal herb rich in tanshinones and salvianolic acids—two compound families with different polarities. Pure SC-CO2 struggles to extract both effectively.
Using 95% ethanol as an entrainer, researchers optimized the following conditions:
- Pressure: 28 MPa
- Temperature: 42°C
- Extraction Time: 3.5 hours
- Ethanol dosage: 0.5 g/g of raw material
Results:
- Extraction yield: 7.9%
- Tanshinone IIA content: 16.2%
- Salvianolic acid B content: 10.7%
Compared to traditional solvent extraction or pure CO₂, the entrainer-assisted process achieved higher purity, stability, and yield, demonstrating the power of entrainers in fine herbal extractions.
Challenges and Considerations
While entrainers improve extraction, they also complicate downstream processing:
- Residual entrainer removal may require evaporation or distillation
- Regulatory approval is necessary for residual levels, especially in food and pharma
- Additional equipment such as entrainer recovery systems may be required
Thus, each application must carefully balance the efficiency gain versus the processing complexity.
Frequently Asked Questions (FAQ)
1. What is an entrainer in supercritical CO₂ extraction?
An entrainer is a small amount of solvent (like ethanol or acetone) added to supercritical CO₂ to improve the solubility and selectivity of target compounds during the extraction process.
2. Why are entrainers important in herbal extractions?
They allow the extraction of polar, bioactive compounds that pure CO₂ cannot extract efficiently, especially important in medicinal herbs like Salvia miltiorrhiza.
3. What solvents are commonly used as entrainers?
Ethanol, methanol, acetone, and benzene (in lab settings) are typical entrainers. Ethanol is most preferred due to its food-grade classification and polarity.
4. How do static and dynamic addition methods differ?
- Static: Entrainer mixed with raw material before extraction
- Dynamic: Entrainer pumped with CO₂ in real-time for better control and efficiency
5. Is supercritical CO₂ extraction with entrainers safe for pharmaceuticals?
Yes, provided residual solvents are minimized and approved solvents are used. Ethanol is generally recognized as safe for nutraceutical and pharmaceutical use.
6. What are the limitations of using entrainers?
- Extra complexity in separation and purification
- Need for solvent recovery systems
- Potential regulatory issues if residues exceed limits
7. Can I use entrainers in essential oil production?
Absolutely. Entrainers enhance yield and component specificity, especially for polar aromatic compounds that are poorly soluble in pure CO2.
Trusted by Global Clients
Our CO2 extraction systems have been sold to over 30 countries, including the USA, Germany, Thailand, and Australia. From start-up brands to well-known herbal extract manufacturers, our customers trust us for:
- Reliable equipment performance
- Excellent technical support
- One-stop turnkey solutions
- Remote installation guidance and training
Supercritical CO2 Extraction Market Report
Contact Us
Looking for the right supercritical CO2 extraction equipment for your business? Contact us today for custom quotes, technical consultation, and free extraction process evaluation.
Email: [email protected]
WhatsApp/Phone: +86-134-8515-5519